GENETRON HEALTH (BEIJING) CO., LTD
Exhibitors
Information
Founded in 2013, Genetron Health is a leading precision oncology platform company in China that specializes in cancer molecular profiling and harnesses advanced technologies in molecular biology and data science to transform cancer treatment. The Company has developed a comprehensive oncology portfolio that covers the entire spectrum of cancer management, addressing needs and challenges from early screening, diagnostics & monitoring, and biopharma development services. Genetron Health is committed to applying innovative genomic technologies to cancer diagnosis and treatment, with the ultimate goal of overcoming cancer.
In June 2020, Genetron Health successfully listed on the NASDAQ stock exchange, marking a milestone as one of China’s pioneering oncology genomics firms to enter the U.S. public market. The company achieved the largest IPO (Greenshoe Option) in the global precision oncology sector at the time. In March 2024, Genetron Health finalized its privatization process and voluntarily delisted from NASDAQ.
Cancer Early Screening
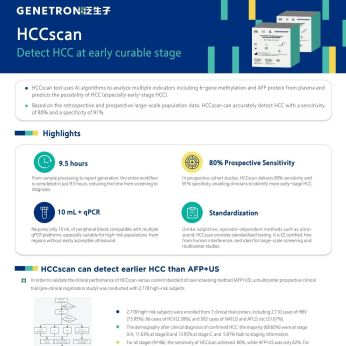
Genetron Health has actively contributed to multiple National Key Research and Development Programs under the guidance of China’s Ministry of Science and Technology, with a focus on advancing diagnostics and treatment for liver cancer, lung cancer, and gastrointestinal cancers. The company has initiated large-scale prospective cohort studies involving over 100,000 participants, demonstrating its commitment to evidence-based innovation. Its proprietary liquid biopsy technology, HCCscreen, designed for early detection of hepatocellular carcinoma (HCC), has achieved global recognition. The groundbreaking research supporting HCCscreen was published in the Proceedings of the National Academy of Sciences (PNAS) and was awarded the prestigious “Breakthrough Device” designation by the U.S. FDA. Furthermore, HCCscreen has been cited in several expert consensus guidelines, including China’s first liver cancer prevention and treatment guidelines. The PCR-based HCCscan is also currently undergoing clinical trials for regulatory approval.
Cancer Diagnostics &Monitoring
Genetron Health’s proprietary “One-Step Seq Method” has positioned the company as a pioneer in precision medicine. This groundbreaking technology was instrumental in securing the Second Prize of China’s National Science and Technology Progress Award — a first for any precision medicine enterprise in China. Complementing this achievement, our Mutation Capsule Plus technology garnered international recognition through its publication in Science Translational Medicine, underscoring its scientific rigor and innovation. Leveraging patented platforms such as the One-Step Seq Method, Genetron Health has built a comprehensive IVD portfolio across NGS, dPCR, and qPCR technologies. To date, 8 instruments and assays have obtained clinical approval from China’s National Medical Products Administration (NMPA), and 11 products are CE-marked for global markets, with additional offerings advancing through development and clinical validation. Our solutions span solid and hematologic tumors, supporting leading hospitals and research institutions nationwide. It has also collaborated with AstraZeneca R&D China to develop a tumor-informed minimal residual disease (MRD) test for various solid tumors, marking the first MRD test co-developed by a precision oncology platform and a biopharmaceutical company in China.
Biopharma Development Services
Genetron Health delivers integrated end-to-end solutions for genomics research and clinical development, spanning biomarker evaluation for targeted and immunotherapies, clinical trial patient recruitment, companion diagnostic development, and CDx registration. With strategic collaborations spanning over 100 leading global biopharmaceutical firms and CROs across China and international markets, Genetron Health is a trusted partner in accelerating precision medicine. Notably, the company pioneered the first NMPA-approved bridging companion diagnostic reagent following the implementation of China’s CDx regulatory guidelines, underscoring its commitment to advancing innovative and compliant diagnostic solutions.