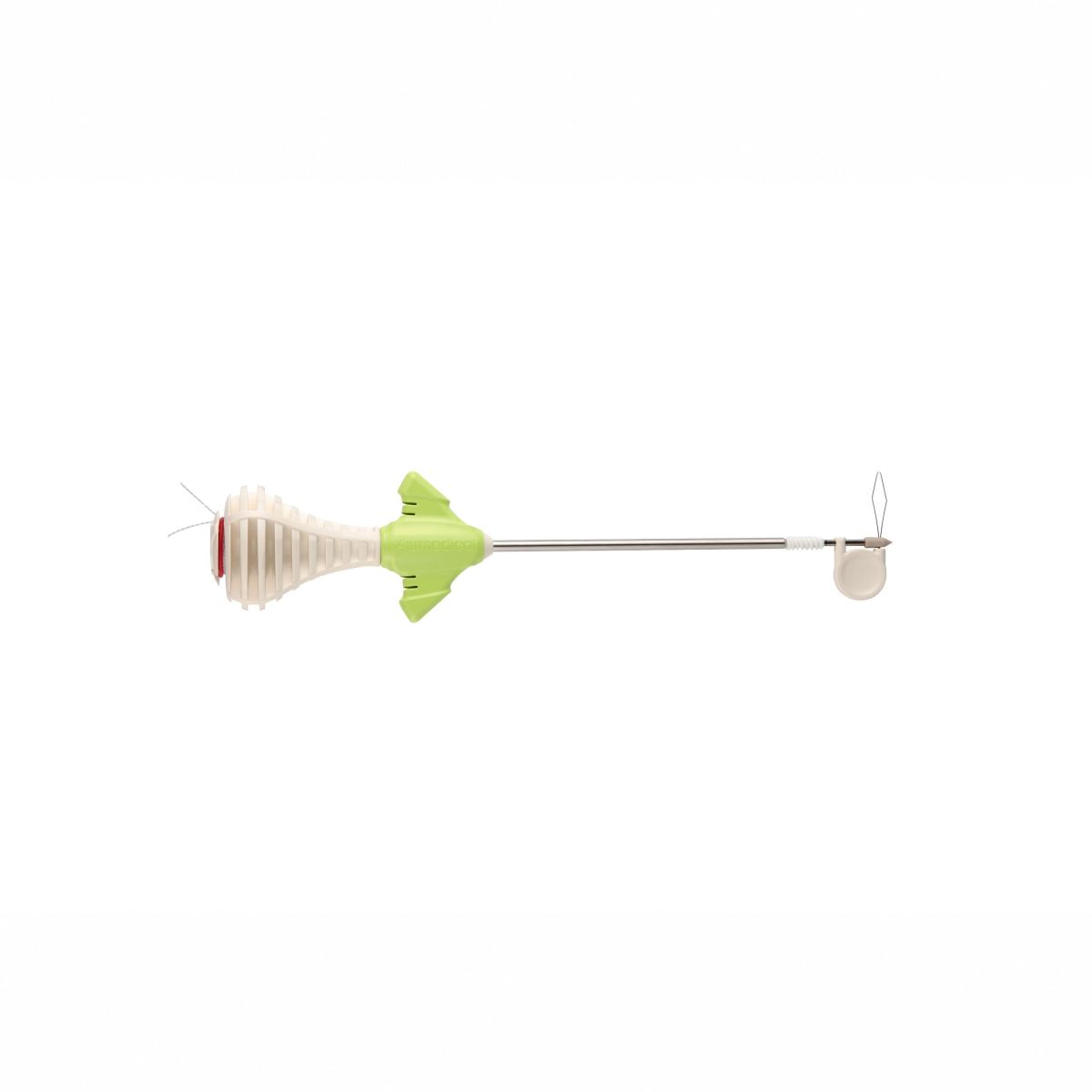
FIXONE HYBRID SUTURE ANCHOR (Fixone KPN)
Implants, Prosthetics
Information
■ Regulatory Status: Korean FDA cleared, FDA cleared (K203523)
■ Material composition: Composed of PEEK tip and biodegradable PLGA copolymer combined with β-TCP screw, supporting gradual resorption and promoting natural bone integration.
■ Various sizes: 4.75mm, 5.5mm
■ Screw-in Design: Provides strong mechanical fixation with dual threading that ensures enhanced anchoring in both cortical andcancellous bone.
■ Knotless Design: Simplifies surgical procedure by eliminating the need for knot tying, reducing operative time and lowering the risk of knot-related complications.
■ Load up to 12 sutures
■ Strong fixation: Offers excellent anchoring strength in both cortical and cancellous bone with optimized thread design for secure tissue fixation.
■ Sterile and ready-to-use